Abstract
Introduction: Arterial and venous thromboembolic events, including stroke and myocardial infarction, are a leading cause of morbidity and mortality in the United States. Anticoagulation therapies are critical in preventing such morbidity and/or mortality. Vitamin K antagonist (VKA) such as warfarin has been the standard therapy for decades until recently with the advent of direct oral anticoagulants (DOACs). These newer agents offer important advantages over warfarin however, the risk of bleeding with these drugs as with all anticoagulants remains an ongoing safety concern. Among the most serious adverse events are intracranial hemorrhages (ICH) and major gastrointestinal bleed (MGIB). For patients who were on anticoagulation prior to these events, there seems to be no consensus on the appropriate timing to resume anticoagulation if at all. Our group reported earlier on the outcome of restarting anticoagulation after ICH (ASH 2017;Oral Abstract #332). Herein, we aim to retrospectively investigate the clinical outcome in terms of recurrent bleeding or thromboembolic events following resumption of anticoagulation post MGIB. Clinical outcome analysis was also extended to compare VKA to DOACs.
Method: We retrospectively reviewed medical records of over 800 adults [mean age 68 (22-81)] at Beaumont Hospital, an academic community center from January 2008 to January 2018 with confirmed MGIB. Patients with MGIB who were not on anticoagulation prior to and after the bleeding event were excluded from study. Patients who were on antiplatelet therapy were also excluded. A total of 280 patients met the criteria. MGIB is defined as decrease in hemoglobin by 2 grams below baseline and/or endoscopically confirmed bleeding. Association between restarting anticoagulation (VKA or DOACs) and re-bleeding or thromboembolic event was made using time-to-event adjusted analyses.
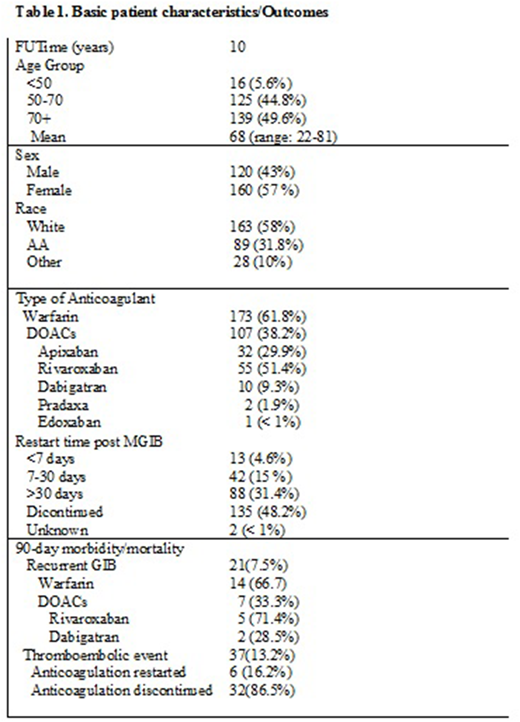
Results: Demographic distribution of studied patients were 163 (58.2%) white, 89 (31.8%) African Americans and 28 (10%) others. Among these patients, 173 (61.8%) were on VKA, 107 (38.2%) were on one of the DOACs (51.4% rivaroxaban; 29.9% apixaban; 9.3% dabigatran; 2% pradaxa; < 1% edoxaban) (Table 1). Although anticoagulation was not reinstituted in 135 (48.2%) patients following MGIB; 13 (4.6%) patients had anticoagulant restarted within 7 days of diagnosis of MGIB; 42 (15%) patients were restarted between 7-30 days and 88 (31.4%) patients were restarted after 30 days. Majority of patients who restarted anticoagulation within 7 days had severe cardiac dysfunction requiring mechanical valves. Among those who restarted anticoagulation within 2 weeks of MGIB, 21 (7.5%) had recurrent GIB; 18 of those patients were restarted within 7 days whereas 3 were restarted between days 7-12. Majority of the recurrent GIB occurred in the warfarin arm since all patients with mechanical valves were on warfarin. Among the DOACS, rivaroxaban rated highest in terms of recurrent GIB (Table 1). The incidence of thromboembolic event was higher among those whose anticoagulants were discontinued after MGIB compared to those who resumed anticoagulation regardless of timing (86.5% vs 16.2%, p < 0.0001).
Conclusion: Given the rising national trend on the use of anticoagulants for various medical necessities, it is imperative that a safe and efficient process be devised on reinstitution of anticoagulation post MGIB to guide Clinicians. Although our study represents a single institutional analysis, it concurs with recent studies that early resumption of anticoagulant following stabilization of MGIB is associated with lower thromboembolic events. Timing for resumption depends largely on the medical reason for anticoagulation; reinstitution by day 7 appear safe for patients on mechanical valve whereas after day 12 maybe appropriate for patients with Atrial fibrillation or venous thromboembolism. Our study further reveals that the risk of recurrent bleed and/or thromboembolism may be less in patients on DOACs when compared to VKA. Among those on DOACs, Rivaroxaban had the highest re-bleeding rate. Nonetheless, randomized clinical trials are needed to evaluate the true risk-benefit profile of anticoagulation resumption after MGIB.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.